Share Links
CENTRAL SLEEP APNEA (CSA): SYMPTOMS, CAUSES, AND TREATMENT OPTIONS
Central sleep apnea is a form of sleep apnea that is less common and less studied than obstructive sleep apnea. This article explains the differences between central and obstructive sleep apnea, as well as the symptoms, causes, and treatment options for central sleep apnea.
What is Sleep Apnea?
Sleep apnea is a disorder where breathing is repeatedly interrupted while asleep. This leads to regular starting and stopping of breath. Sleep apnea is associated with a variety of negative short-term and long-term effects.

Types of Sleep Apnea
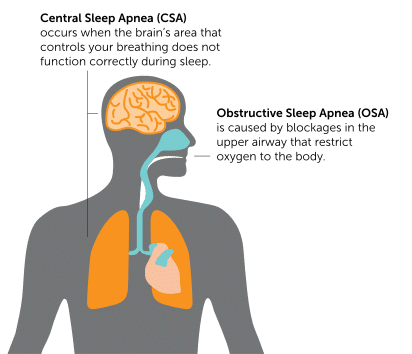
Sleep apnea is usually classified as either obstructive sleep apnea (OSA) or central sleep apnea (CSA). The classification is determined based on the more predominant type of event.1 Often people with sleep apnea have a combination of both obstructive and central events.
OBSTRUCTIVE SLEEP APNEA (OSA)
Obstructive sleep apnea (OSA) occurs when the body tries to breath, but a narrowing or obstruction of the breathing passages prevents air from flowing freely. The obstruction can be due to bodily features or a relaxation of the muscles in the chest and neck during sleep.2
CENTRAL SLEEP APNEA
Central sleep apnea (CSA) occurs when the brain fails to signal the breathing muscle (diaphragm) to activate. As a result, the person either “forgets” to breathe or breathes less deeply than normal.3 Notably, “pure” central sleep apnea (no obstructive events) is uncommon. Central Sleep Apnea generally refers to patients who have more central events than obstructive events.
OTHER TYPES OF SLEEP APNEA: MIXED AND COMPLEX SLEEP APNEA
What is Central Sleep Apnea?
Central Sleep Apnea is defined as “the temporary withdrawal of central (brainstem-driven) respiratory drive that results in the cessation of respiratory muscle activity and airflow.”5 To better understand this definition, it is helpful to compare central sleep apnea with normal breathing. In a healthy patient, the brain signals the breathing muscle (diaphragm) to contract at regular intervals. The signal to breathe travels from the brain stem down the phrenic nerve and then to the diaphragm, causing a contraction. This contraction of the diaphragm pulls air into the lungs. For patients with central sleep apnea, the brain fails to send regular signals to the diaphragm. The result is an extended “pause” of breathing, ranging in duration from roughly 10 to 40 seconds. An episode may result in:
- Sleep disturbance
- Decrease in the body’s oxygen levels (also known as hypoxia)
- Surge of nor-epinephrine (the body’s “fight or flight response,” which may feel like a racing heartbeat).
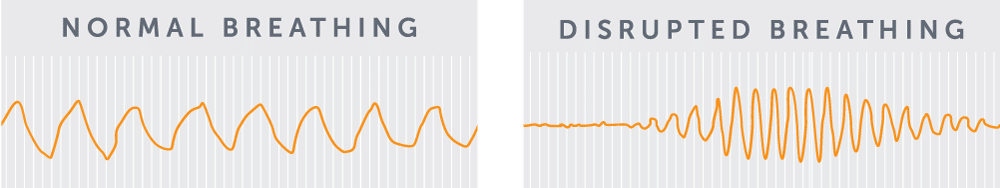
These events can happen multiple times per hour—sometimes as frequently as once per minute. However, these sleep disturbances are often not enough to fully wake the patient. A patient’s sleep partner may actually be more likely to identify the breathing disorder than the patient himself/herself!
Symptoms of Untreated Central Sleep Apnea
Central sleep apnea has symptoms that are both long term and short term. Symptoms of central sleep apnea (CSA) include:2,6
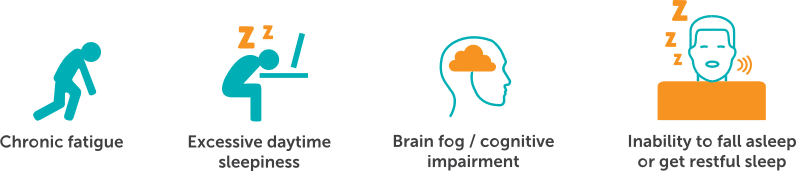
Many patients with CSA also have heart disease, especially heart failure.7 Within this population, patients with CSA are at increased risk for hospitalizations and even death.8,9
Central Sleep Apnea Causes and Associations
Central sleep apnea is caused by a failure of a person’s brain to consistently send signals to activate the breathing muscles while asleep. Central sleep apnea has a number of known associations, including2
- Cardiac disorders, including congestive heart failure (CHF) and atrial fibrillation (AFib)
- High altitude
- Opioids
- Treatment emergent central sleep apnea
- Idiopathic central sleep apnea
- Other medical conditions (e.g. stroke, brainstem/spinal cord disorders)
CARDIAC DISORDERS
Studies have shown that up to 40% of people with congestive heart failure (CHF) and 30% of people with atrial fibrillation (AFib) may have central sleep apnea.10,11 In fact, heart failure is the most common cause of CSA in the general population.2
HIGH ALTITUDE
Periodic breathing appears when people are exposed to high altitudes. This may be due to how your body responds to changes in the atmosphere.2
OPIOIDS
TREATMENT EMERGENT CENTRAL SLEEP APNEA
Treatment emergent central sleep apnea refers to central events that occur when a person is using Positive Airway Pressure (PAP) therapy such as CPAP or BiPAP. Reasons for treatment emergent CSA may occur due to an increased number of arousals during titration, mask leak, or overtitration. This may improve with ongoing PAP use.2
IDIOPATHIC CENTRAL SLEEP APNEA
In some cases, an underlying cause or association for central sleep apnea cannot be identified. This is rare in the general population and is generally diagnosed by ruling out other causes.2
Treatment Options
IMPLANTABLE PHRENIC NERVE STIMULATION (THE remedē SYSTEM)
Phrenic Nerve Stimulation (brand name remedē®, www.respicardia.com) is an FDA-approved, implantable, non-mask therapy for moderate to severe central sleep apnea in adult patients. The device activates automatically each night to send signals to the breathing muscle (diaphragm) via the phrenic nerve to restore a normal breathing pattern. It monitors respiratory signals while you sleep and helps restore normal breathing patterns. Because the device is implantable and activates automatically, it does not require wearing a mask; however, as with any implantable device procedure, there is a risk of implant site infection.12
POSITIVE AIRWAY PRESSURE (PAP) THERAPY
Positive airway pressure devices (e.g. brand names AirSense™, DreamStation, www.resmed.com, www.respironics.com ) are often used to treat central sleep apnea. Various types exist, including those that provide continuous pressure (i.e. CPAP) and those that provide variable pressure (e.g. BiPAP, ASV). Notably, ASV, or adaptive-servo ventilation, is contraindicated (i.e. should not be used) in patients with chronic, symptomatic heart failure with reduced left ventricular ejection fraction (LVEF ≤ 45%).11
SUPPLEMENTAL OXYGEN
Physicians may recommend the use of supplemental oxygen for patients with central sleep apnea. Various options can be used to deliver oxygen to the patient.13
PHARMACOLOGICAL
Pharmacological agents such as acetazolamide and theophylline have been attempted as therapies for central sleep apnea. However, limited data has been collected for the efficacy and safety of these therapies.
Benefits of Treated Central Sleep Apnea (CSA)
Restorative sleep is important for our physical and mental wellbeing. Addressing central sleep apnea may improve a person’s sleep breathing, sleep quality, and quality of life.
Summary
Central sleep apnea is a form of sleep apnea that occurs when the brain fails to send regular signals to the breathing muscle (diaphragm) during sleep. Symptoms include daytime sleepiness, fatigue, and an inability to get restful sleep. Untreated central sleep apnea can also have negative long term effects on a person’s health. Treatment options are available for central sleep apnea. Consult your doctor if you think you may have central sleep apnea and would like to learn more.


COULD THE remedē SYSTEM BE RIGHT FOR YOU?
Answer a few short questions to see if you may be a candidate.
Important safety information
The remedē® System is indicated for moderate to severe Central Sleep Apnea in adult patients.
Your doctor will need to evaluate your condition to determine if the remedē system is right for you. You will not be able to have an MRI or diathermy (special heat therapies) if you have the remedē system implanted. The remedē® System may be used if you have another stimulation device such as a heart pacemaker or defibrillator; special testing will be needed to ensure the devices are not interacting.
As with any surgically implanted device, there are risks related to the surgical procedure itself which may include, but are not limited to, pain, swelling, and infection.
Once the therapy is turned on, some patients may experience discomfort from stimulation and/or from the presence of the device. The majority of these events are resolved either on their own or by adjusting the therapy settings. The remedē® System may not work for everyone. There are additional risks associated with removing your system. If you and your doctor decide to remove the system, another surgery will be required.Be sure to talk with your doctor so that you thoroughly understand all of the risks and benefits associated with the implantation of the remedē® System. Rx only. For further information, please call +1-952-540-4470 or email info@respicardia.com.
- A Report of the American Academy of Sleep Medicine Task Force. Sleep-related sleep disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. SLEEP 1999;22:667–89.
- Javaheri S., Dempsey J.A. (2013) Central sleep apnea. Compr Physiol 3:141–163.
- Dempsey J.A., et al. Pathophysiology of Sleep Apnea. Physiol Rev 90:47-112, 2010. doi:10.1152/physrev.00043.2008.
- Morgenthaler T; Gay P; Gordon N et al. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. SLEEP 2007;30(4):468-475.
- Costanzo M.R., Khayat R., Ponikowski P., et al. Mechanisms and clinical consequences of untreated central sleep apnea in heart failure. J Am Coll Cardiol. 2015; 65:72–84.
- Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol 90: 13–24, 2005.
- Bekfani T, Abraham WT. Europace. 2016 Aug;18(8):1123-34. doi: 10.1093/europace/euv435. Epub 2016 May 26.
- Khayat R et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012;18:534–40.
- Khayat, R et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure, European Heart Journal 2015;36 1463–1469.
- Bitter T, et al.. Dtsch Arztebl Int. 2009;106(10):164–170.
- Costanzo MR, et al. J Am Coll Cardiol 2015; 65:72–84.
- FDA PMA P160039 https://www.fda.gov/medical-devices/recently-approved-devices/remeder-system-p160039.
- Aurora RN et al. Updated adaptive servo-ventilation recommendations for the 2012 AASM Guideline. J Clin Sleep Med. 2016;12:757-61.

