IMPROVE
YOUR QUALITY OF LIFE
IMPROVE
YOUR QUALITY OF LIFE


The remedē® System is a breakthrough implantable system that safely and effectively treats moderate to severe central sleep apnea (CSA) in adult patients.3 CSA is a serious breathing disorder that disrupts the normal breathing pattern during sleep and has been shown to negatively impact quality of life and heart health.1
The latest advancement in remedē System technology is now FDA approved.
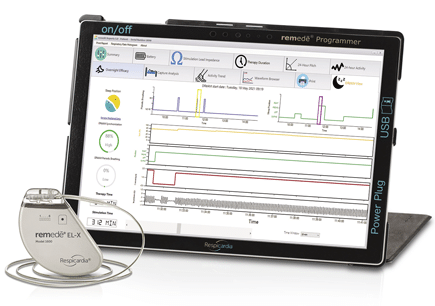
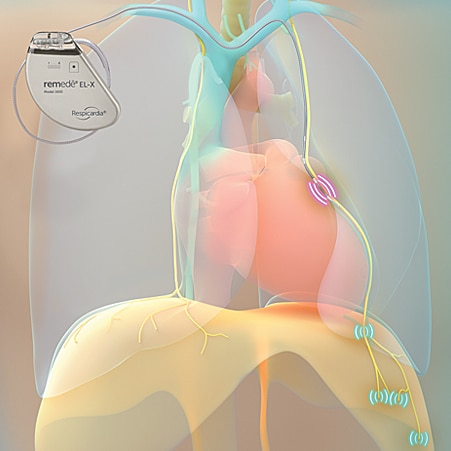
The remedē System is an implantable system that stimulates a nerve in the chest (phrenic nerve) to send signals to the large muscle that controls breathing (the diaphragm). These signals stimulate breathing in the same way that the brain signals breathing.
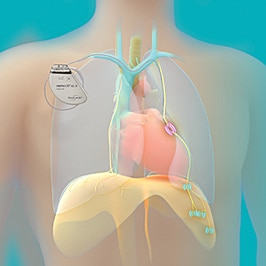
improve sleep
How it works
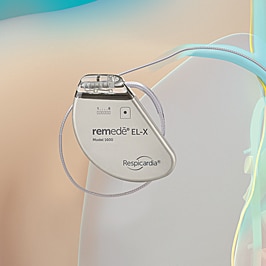
The remedē System is placed during a minimally invasive outpatient procedure by a cardiologist. The system is a battery powered device placed under the skin in the upper chest area with two small thin wires (leads), one to deliver the therapy (stimulation lead) and one to sense breathing (sensing lead).
The remedē System works to continuously and automatically monitor and stabilize the breathing pattern, restoring sleep throughout the night.
improve sleep
How it works



clinically proven
In a clinical study, the remedē System has been shown to significantly reduce the effects of CSA:
91% of patients had a
reduction in the number of
sleep apnea events per hour2
82% of patients had an
improvement in quality
of life2
95% of patients would
get remedē® again2
At 6 months, 48% of the control group had a positive change in apnea events per hour and 13% of the control group had an improvement in quality of life.
COMMONLY ASKED QUESTIONS
The remedē System is approved for adult patients with moderate to severe central sleep apnea. Your doctor will need to determine your type and severity of sleep apnea. Your doctor will need to determine the type and severity of sleep apnea based on your sleep study. If your doctor determines that you have central sleep apnea you should discuss with your physician if the remedē System is right for you. The remedē System reduces the number of sleep apnea events at night and daytime sleepiness and improves sleep quality and quality of life, as assessed by patients.3
The remedē System is a treatment option for adult patients with moderate to severe central sleep apnea. The device is an implantable system which uses stimulation of a nerve in the chest (phrenic nerve) to send signals to the large muscle between the chest and abdomen (the diaphragm). These signals stimulate breathing in the same way that the brain signals breathing.
This treatment starts and stops on its own and does not require you to wear anything on your face. No equipment is needed at home.
The remedē System implant is typically an outpatient procedure that is performed under light sedation. Patients usually stay awake, but are drowsy during the procedure. You may stay in the hospital overnight, which will depend on your health, how well you tolerate the procedure and how fast you recover. Please consult with your doctor for additional information.
As with any surgically implanted device, there are risks related to the implant procedure which may include, but are not limited to, pain, swelling and infection. Once the remedē System is implanted and the therapy is turned on, some patients may experience discomfort from stimulation and/or from the presence of the device. The majority of these events are resolved on their own or by adjusting the therapy settings. The remedē System may not work for everyone. There are additional risks associated with removing your system. If you and your doctor decide to remove the system, another surgery will be required.
Be sure to talk with your doctor so that you thoroughly understand all of the risks and benefits associated with the implantation of the remedē System.
About one month after your procedure, therapy will be started. Over the first three months of therapy, you will work with your doctor to ensure that the therapy is set up for your individual needs. Once the therapy is adjusted for you, you will need check-up visits every 3-6 months. In the first few months after the therapy begins, you may be aware of the movements of your diaphragm at night. This shows that the therapy is active. Most patients adjust to the therapy within the first 3 months.
The remedē System should not be placed if an active infection is present.
The remedē System cannot be used with magnetic resonance imaging (MRI). MRI imaging is a non-invasive test that doctors may use to diagnose various conditions such as strokes, cancer, and joint problems. Contact your doctor with questions regarding the use of MRI and the remedē System.
You can return to most of your normal routine within a week. You will be asked not to lift your arm above shoulder height for typically 1-3 months after the implant procedure.

HEAR FROM PEOPLE LIKE
YOU
People share how they decided to get the remedē System and how it’s changed their lives.
*Required to distinguish the type of sleep apnea.
- Costanzo M.R., Khayat R., Ponikowski P., et al. Mechanisms and clinical consequences of untreated central sleep apnea in heart failure. J Am Coll Cardiol. 2015; 65:72–84.
- Costanzo M, et al. Sustained Twelve Month Benefit of Phrenic Nerve Stimulation for Central Sleep Apnea. Am J Cardiol2018;121:1400-8.
- Costanzo M, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. The Lancet. 2016; 388: 974–82.


