The Valley Hospital Introduces Sleep Apnea Advance
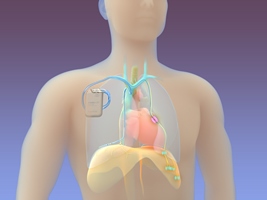
The Valley Hospital is one of only two sites in New Jersey and the tri-state area currently using a new implantable device to treat adult patients with central sleep apnea.
Central sleep apnea is a serious condition in which a person’s sleep is interrupted because the brain does not send proper signals to the diaphragm during sleep, causing lapses in breathing.
The remedē® System, approved by the FDA in 2017, is a pacemaker-like device that is placed under the skin in the upper chest area during a minimally invasive outpatient procedure.
It treats central sleep apnea by activating a nerve located in the chest that stimulates breathing. The system monitors the patient’s breathing during sleep and if irregular breathing is detected, it stimulates the nerve to move the diaphragm and restore normal breathing.
“The new remedē® System provides physicians with an innovative therapy for patients with moderate to severe central sleep apnea,” said Dr. Suneet Mittal, Director of Electrophysiology at Valley and Medical Director of Valley’s Snyder Center for Comprehensive Atrial Fibrillation. “This therapy has been proven to reduce the number of sleep apnea events, which will improve patients’ quality of life and overall cardiovascular health.”
Dr. Mittal and cardiac electrophysiologist Dr. Dan Musat performed the first remedē® procedures at Valley, in consultation with a multidisciplinary team that included heart failure specialist Dr. Kariann Abbate, and Dr. Jeffrey Barasch, Medical Director of The Valley Hospital Center for Sleep Medicine.
Central sleep apnea causes significant drops in night-time blood oxygen levels and disrupted sleep, with significant impairment in cardiac function and daytime performance. The condition is different from the more well known obstructive sleep apnea, in which breathing disruptions occur when the throat muscles intermittently relax and block the airway during sleep.
Until this new development, central sleep apnea has been difficult to treat, frequently resistant to medical therapy and unresponsive to CPAP, the usual therapy for obstructive sleep apnea.
“Treatment for this particular type of sleep apnea has been a significant challenge over the years,” said Dr. Barasch. “We believe that his new technology is a major step forward that will enable us to treat these patients much more effectively.”
The remedē® System is produced by Respicardia Inc., based in Minneapolis, Minn.
To make an appointment for a consultation to see if you are a candidate for the remedē® System, please call 201-447-8392.

